December 1, 2008
Off Label Prescribing Turns Out To Be On Label
In which I bite down on a leather strap in an effort not to explode like that guy on Heroes.
An article in Pharmacotherapy that defies credulity. Really. Just look at me. I'm incredulous.
I. "These Drugs Are Not Indicated For The Following:"
The study seeks to examine off label prescribing so that further research into their efficacy and safety can be conducted. No word on who will be paying for that research, which, as I will now demonstrate, is completely unnecessary.
Here's the summary: looking at prescriptions versus indications, the authors found a list of drugs with substantial off-label use in the absence of substantial evidence for their use. One might ask whether the the substantial off-label use is in itself a form of evidence for its use, but we'll leave that for another day.
Here's the list.
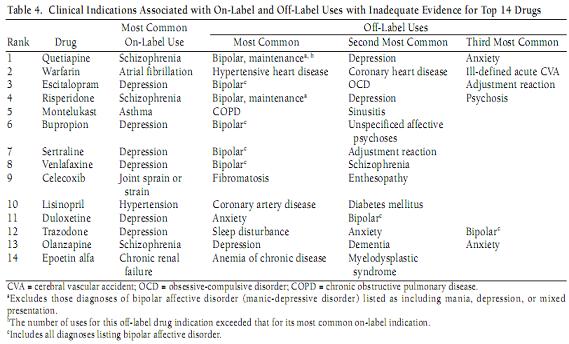
IIa. I Thought It Was Indicated For That?
Right off the bat, you'll notice that Seroquel's most common off label use is bipolar, which is doubly wrong, because it is actually indicated for bipolar; and bipolar, not schizophrenia, is actually its most common on-label use.
Worse, this study is about use without "adequate evidence supporting its use" (look at the title of the chart.) So either these authors were completely unaware of the studies supporting its use and labeling by the FDA; or they were aware of it, and didn't think it was as adequate as the FDA apparently did. Either way, someone needs to quit drinking.
Next, While it is true that these drugs are being used off-label, they aren't being used off-label randomly. According to the study, Seroquel is used on label, or for the top three off-label uses, 77% of the time. Lexapro it's 98%. They're not using Seroquel for asthmatics. The issue isn't whether "depression" off-label use exceeds "anxiety" off-label use; it's whether there is any difference between either of those two uses. There isn't.
IIb. What Is An Indication?
For those who are not in the biz, it may surprise you to learn that the FDA does not decide what a drug is indicated for. It only decides if the indication that Pharma chooses to request is approved or not. If Risperdal is approved for bipolar mania but not maintenance, it doesn't mean that it isn't safe and efficacious for maintenance; or that there isn't a huge collection of data affirming this. It means Janssen didn't ask the FDA for the approval. In other words, 95% of the decision for an indication is made by the folks down at Pharma marketing. All the FDA can do is say yes or no.
And, as evidence, let me now say, categorically, that Risperdal will never get a maintenance indication. Not because it isn't efficacious or safe-- who knows?-- but because it's generic: no one will pay to get it approved.
III. "Oh, No, Doctor, You Need To Be Rigorous."
You'll notice a little superscript "a" which says that if bipolar was diagnosed along with mania or depression, then that wasn't counted as off-label. In other words, simply writing "bipolar" was considered off label? Yes.
But what about maintenance? Isn't it indicated for bipolar maintenance?
If you want to be technically accurate, Seroquel isn't indicated for bipolar maintenance-- it's indicated as an adjunct to lithium or Depakote for maintenance. How would the authors have known if it was being given with Depakote? They wouldn't.
You will notice, however, that Depakote is not on this list, despite it's not being indicated for anything except mania. All those Depakote scripts are all for acute mania? Wow.
(BTW, can you force Seroquel to be given as an adjunct to something else, when the somethiing else is itself neither approved nor effective? Hello?)
I'm splitting hairs, you think? Nope. It matters, because this information found it's way into the news media:
From USAToday:
Seroquel, an antipsychotic approved for treating schizophrenia and short-term manic or depressive episodes in bipolar disorder, topped the list. Three out of four times, doctors use the drug off-label, mainly for maintenance therapy for bipolar disease, says senior author Randall Stafford, associate professor of medicine at the Stanford Prevention Research Center.
Got that? According to USAToday and Dr. Stafford, Seroquel isn't approved for bipolar maintenance. "Permission to get angry at Big Pharma?" "Permission granted. Fire at will."
IVa. There Is Method To This Madness
But arguing about the results of a study is freshman level sophistry. The real money is in the Methods section:
Question: How do they know what a drug was being used for, that they could call it off-label?
Answer: They took the data from a self-report survey of doctors. When he wrote "Seroquel" did he also write "bipolar?"
How do you know that what he calls "bipolar" is really bipolar? If he calls it bipolar, and uses Seroquel, but it's actually anxiety, is that on-label or off-label? According to this, it's off-label. So, in other words, on/off-label is defined not by reality, but by whether the doctor explicitly provides a label. Not whether he is right, but confident.
Then what prevents a doctor from simply writing bipolar every time he wants to use Seroquel?
Etc.
IVb. I Want To See This Self-Report Survey
If the lack of rigor in the psychiatric diagnoses isn't bad enough, how about this: how do you know the self-report isn't completely made up?
I don't mean made up as a "cover your ass"-- that the doctor writes "bipolar" just to justify his Seroquel; I mean that he completely invents the patient data?
I looked up their survey. Fortunately for me it's online; unfortunately for any doctor filling it out, it therefore takes forever to complete:
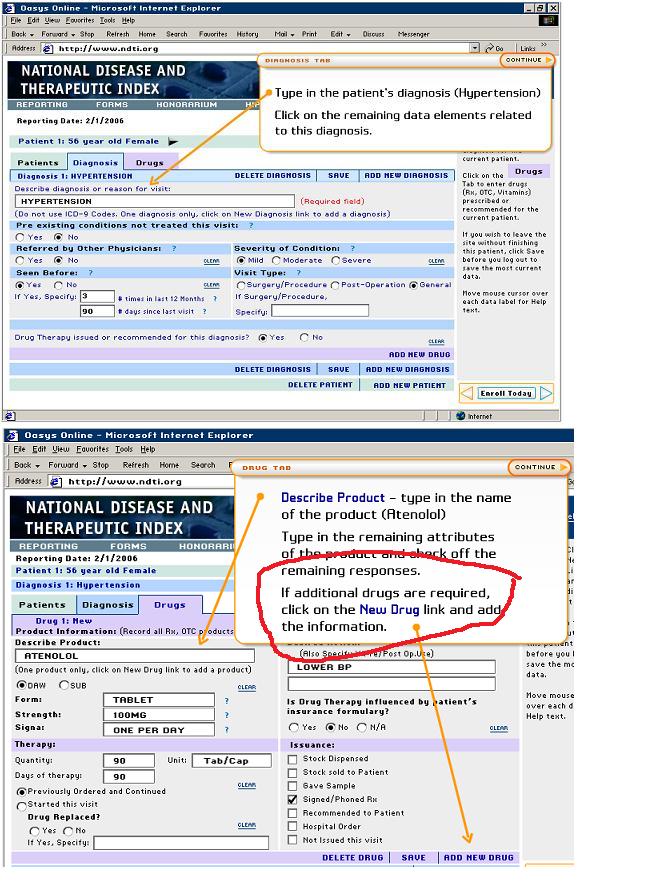
As you can see, there are three different screens: one for patient demographics (age, insurance, etc); another fopr diagnosis; and another for drugs. The doctor needs to type in the name of the drug, and also what he used it for. There is no accountability. There is no incentive to write "Major Depressive Disorder" or "Bipolar Disorder, Mixed state." You would simply write "depression" or "bipolar." Hell, there's not even an incentive to diagnose "obsessive-compulsive disorder" since "anxiety" would just about cover it.
Now go look at that superscript "a" and tell me if it's significant.
Also note that you fill out a whole new screen for every diagnosis (e.g. one for MDD, one for alcohol abuse, one for...) and each drug. Imagine you have 8-50 patients a day, two or three meds per patient (not including drugs from other providers for other problems that you are still responsible to enter), one or two diagnoses per patient...
All this might be filled out correctly, or it might not. Or maybe suddenly everyone got diagnosed with "MDD." Maybe everyone got "effexor" but not "effexor xr." There is no way of knowing.
V. Are You Saying This Study Is Invalid?
Unreliable diagnoses; unreliable application of those diagnoses; and unreliable self-report of those unreliable diagnoses that were unreliably applied, along with unreliable report of the medications used. Peer review x 3, and publish.
But don't run off just yet, Ironman. This doesn't mean that this particular study is invalid. It means all studies based on psychiatrist self-report are invalid. All of them. Write that down. All of them.
VI. "You're Being Unfair."
"But just a minute, Dr. Pirate, you aren't being fair. Seroquel received approval for bipolar maintenance in May 2008. The study used June 30, 2007 as its cutoff for FDA approvals."
Yes. That's not suspicious to you?
And even if that was an accident of history-- the study was completed before Seroquel got the maintenance indication-- why wouldn't they go back and revise the manuscript which was published only two weeks ago? Wouldn't you at least mention this new development in the news interviews you gave? This is supposed to be science. Do you allow the publication of a study when you now know that the main result of your study is wrong?
You know, so as not to look like you're purposely generating a negative article about something which you now know to be untrue?
VI. Who Was This Study For?
It's for politics, of course. The study is published in Pharmacotherapy so that it can be written up in the Wall Street Journal, or USAToday; so that they can be interviewed. It is a MacGuffin. Doctors aren't reading it, the public is reading it. It's war by other means.
It also shifts money around, the historical purpose of government. It sounds the alarm of too much spent on marketing, too little on safety and efficacy. In the era of bailouts, this is an excellent position to take.
8 Comments