April 28, 2010
Deconstructing a Promotional Slide Deck: Geodon

You can complain that all data is biased and all promotional programs propaganda, but until someone comes up with a better system of medical education and figures out how to pay for it, this is all we have.
My intention is not to do a Carlat "all medicines suck" style hit piece. I want to show how to do a powerful analysis of a PROMOTIONAL slide presentation, and also how to detect some of the typical tricks/misdirections. These are frequently used by academics as well.
These slides are for Geodon's new indication, Geodon as an adjunct to Lithium or Depakote for Maintenance of Bipolar Disorder. It is what doctors (will) see at promotional programs (e.g. dinners) and versions of these will become rep detail pieces (what they'll show doctors.)
I don't have the slides, but I did get a peek at them, so I've redrawn them from memory. Yes, memory.
Slide 1:

Indications ("antipsychotic") etc, are descriptors, not identifiers. 15 years ago Geodon was tested for schizophrenia, and found to be effective. Today, it is tested for maintenance bipolar; so we say "this antipsychotic is also effective for bipolar maintenance." Wrong. That's an accident of history. 15 years ago they could have first tested it for bipolar, and today done schizophrenia trials, and then we'd say, "this is a mood stabilizer that also treats psychosis." Both of those statements are empty. It is a chemical, it has utility, not identity.
Side 2:

What does the author want to be true?
This is the main slide, disguised as a throwaway. As the "introduction" it clarifies for you what is established already-- according to the presenter-- even though it may not actually be established. "As has already been established, Windows is a superior operating system."
Pfizer has decided to market Geodon for mild states, and emphasize it's better weight profile.
Their message is: "hey, even though you think Geodon is weak, nothing works as monotherapy. And we at least have an indication for maintenance."
It seems there's no way to manipulate this: two simple quotes. The second quote seems to follow from the first, i.e. because the rate of success of monotherapy is so low, therefore you need a combination. But, in fact, reference two doesn't attribute the prevalence of combination therapy to a lack of efficacy. The sentence preceding quote 2 is:
There's combination out of necessity, and combination out of availability.
Slide 3:
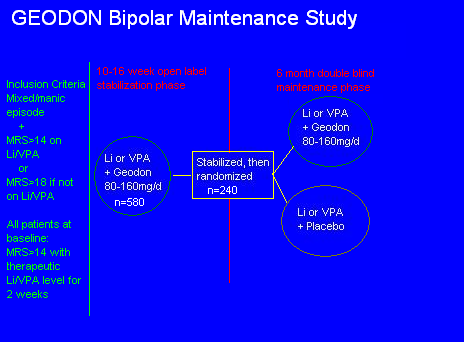
Their message: "We took some manic/mixed bipolar patients, and randomized them to mood stabilizer alone vs. mood stabilizer + Geodon, for 6 months, to see which kept them stable (time to intervention) longer."
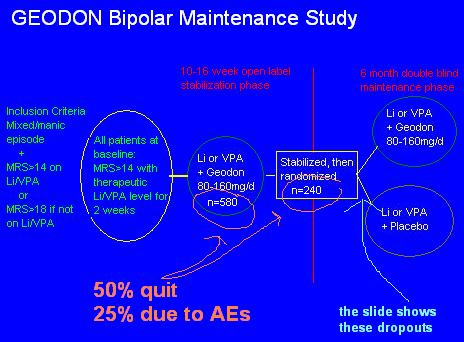
Let's work from the middle "Stabilization/Randomization" square. What were they stabilized with? Li or VPA, + Geodon. What were they randomized to? Either a continuation of that, or the Geodon was taken away. So the study doesn't measure maintenance efficacy, it measures the speed of relapse if you take one of the two medicines that got you stable away.
Go back one step to the first circle. What happened to some of the 580 people who couldn't get stabilized, after 16 weeks, on Li/VPA + Geodon? They didn't stay in the study. Same with those who had some adverse event. 50%, gone. So by the middle square, the study had effectively selected for those patients who could tolerate and respond to Geodon.
Continuing backwards, it appears that the study begins at the big green vertical line, but it doesn't. Reformatting the slide gives a more clear explanation of the events:
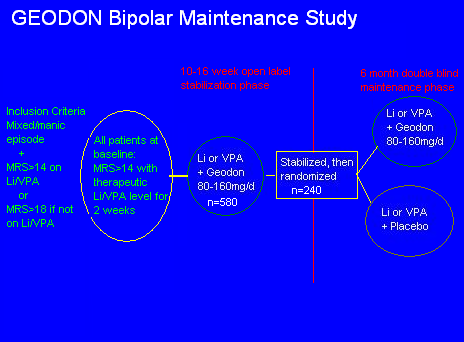
You now can see that there was one extra initial step. Real outpatients on any kind of medications were screened. If they were manic (MRS>14 or 18) then they were TAKEN OFF THEIR MEDS and given Li or VPA, at therapeutic level, for 2 weeks. Those patients who DID NOT IMPROVE on the monotherapy were then given Geodon as well, for 3-4 months, until stabilized.
This effectively screens out patients who respond to monotherapy. Consider: you already know that by the end of the study, the monotherapy patients will relapse faster, because monotherapy didn't work in the first place.
Aside: this may be a question of severity, but how is it possible to let more than 600 people stay manic for two weeks on one single medication, and not intervene?
Slide 4:
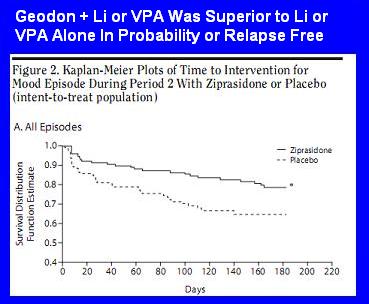
Their message: "Geodon + Li or VPA was superior to Li/VPA alone for preventing relapse."
More full message: "Geodon + Li or VPA significantly increased the probability of being relapse free."
Of the 240 patents who started the maintenance phase, only 138 finished. Kaplan-Meier analysis is a way of analyzing the probability of an event (in this case, being relapse free) even when there are dropouts for other reasons. It's probably more useful to simply say this:
You might be tempted to say the following: "hey, Depakote or lithium alone wasn't that bad-- 70% probability of being relapse free. So?"
What would be awesome was to have a straight placebo arm (i.e. Li/VPA + Geodon vs. Li/VPA + placebo vs. no meds at all), to compare.
Though one shouldn't compare across studies, etc, etc, compare the above survival curves to those found in the famous year long Depakote maintenance study (Depakote vs. lithium vs. placebo monotherapy trial for prevention of mood episodes-- (done by the same guy, hey. would you look at that))
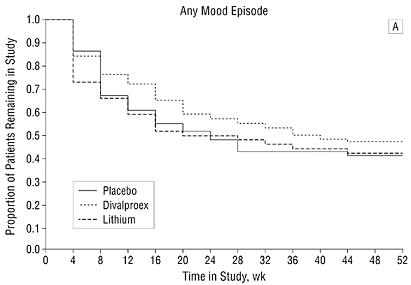
In that study, the same maintenance power of depakote and lithium wasn't better than placebo.
Slide 5: Dosing
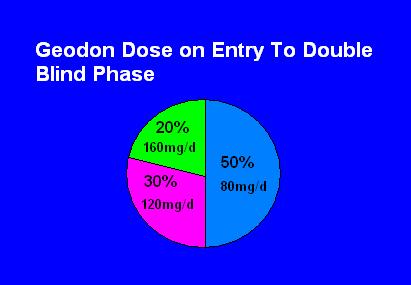
This slide does not tell you the doses used to keep people stable for 6 months; it tells you the doses used to stabilize the patient, i.e. the acute mania dosing.
It is true that they were then kept on these doses, but you don't know if doubling the dose-- or halving the dose-- would have changed the outcome of the maintenance phase.
It is already established in the acute trials that it takes 120-160mg of Geodon ALONE to treat acute mania; perhaps the 50% that only needed 80mg here had some effect of the VPA/Li; or there was better attention to giving with food here; or the permitted use of Ambien and Ativan (<2mg) also helped. In any case, it seems reasonable to say that one should not expect any acute efficacy at all under 80mg.
Slide 6: Nonadherence

Looks like a throwaway; but it's the whole marketing message. Weight gain= Zyprexa/Seroquel; Sedation = Seroquel; Akathisia = Abilify as defined in the secret Big Pharma Marketing Playbook. They all use the same copy. (Geodon would be QTc.) The slide lets you know that this is why patients stop their meds, so be proactive...
First, the actual Guidelines reviewed studies that find that denial of illness and lack of efficacy as the main culprits. In fact, they write "there is not a robust association between medication side effects and adherence... a survey of 3000 patients found that side effects ranked 7th on a list of concerns..."
But those studies aren't the point: the Expert Consensus Guidelines aren't a review of studies, they are a 40 question survey given to 40 experts in bipolar. "What do you think is the standard of care? What's your favorite Dr. Who episode?" Also note that these experts don't routinely treat patients.
The Experts rated side effects as the main adherence issue.
Even more interesting is which side effects these Experts rated as most important, in decreasing order:
Final point:the studies found that patients were most troubled by weight gain and cognitive side effects:
Slide 6: Weight Gain
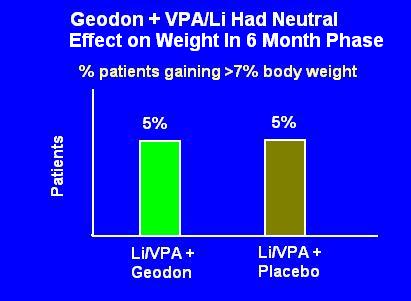
It looks like Geodon had no effect on weight; alternatively, it looks like Li or VPA will cause 5% of patients to gain >7% of their body weight (e.g. 10-15lbs.)
But this tracks weight changes starting from the double blind phase. Remember, patients were loaded with VA or Li for two weeks, then Geodon was added for 10-16 weeks. Not to mention they were already on meds in their past. Could they all have gained 100 lbs in the first four months, only to level off in the maintenance phase? Of course. Did that happen? Who knows?
All that you can say is that after being on Geodon for 4 months, the proportion of people who go on to have even more weight gain in the next 6 months is 5%.
Slides 7 and 8: Sedation and Movement Disorders
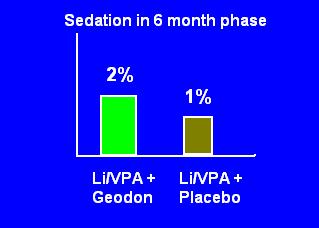
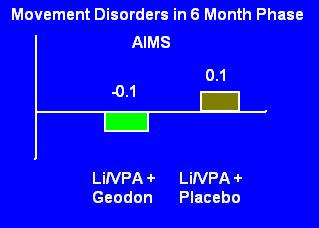
Same deal: these are the side effects only in the last 6 months of a 10 month study. Could sedation have been massive but transient in the first four months? Could tolerance to the effects have developed? We know 50% of patients dropped out by the randomization phase-- half of those due to adverse events. Did the people who experienced sedation or movement disorders quit?
The study had effectively screened for patients who could tolerate these kind of side effects, so you'd expect them to be low in the last half of a study.
Slides 9 and 10: Discontinuation/Tolerability
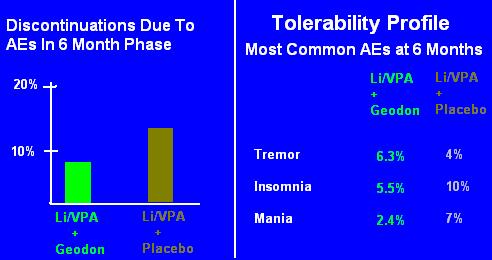
Same deal, again.
Please take a moment and look at the slides as if it was your first time. Despite the clear labeling "At 6 Month Phase", you can't help but think "rates of adverse events were low."
If you're astute, you might even think, "weird, people on two medications actually had fewer side effects, lower discontinuation rates." And your mind would start speculating: well, the Geodon is actually a little activating, so it counteracted the sedation that was caused by the Depakote."
No. The Geodon doesn't counteract the sedation; nor are there really "low rates" of discontinuation. Remember, 50% of the people stopped the combo even before randomization, and 50% of those stopped specifically because of an adverse event.
So Slides 9 and 10 are showing you the rates of AEs in people who had already tolerated 4 months of both medications.
This is important and must be understood. Content-- whether it be a Pharma slide or a newspaper article or anything else-- is almost never factually inaccurate. But the story, the style, the presentation is intended to get you to lie to yourself. This slide very obviously says, "in the 6 Month Phase." There's no misinterpreting that-- and yet you did.
Don't blame yourself entirely, it is a trick. If the slides were presented with the intention of imparting information (instead of a story) then it would never have shown you only the Phase II data, it would have offered you something like this:
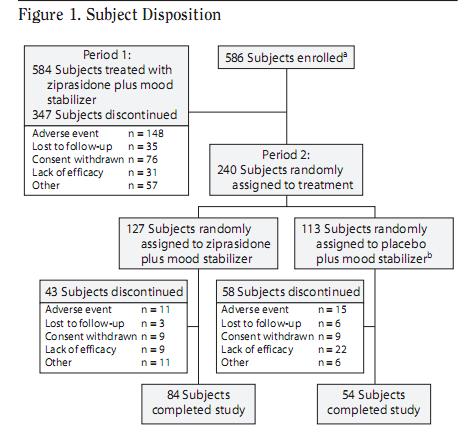 which comes from the study itself.
which comes from the study itself.
But don't get excited, the studies are almost never more honest than the Pharma slides. The above Subject Disposition tree is a very recent phenomenon in articles, forced on it by an exhausted readership, and many articles still don't use it. Not that it would make any difference: no one reads the articles anyway.
Slide 11: Geodon Must Be Taken With Food
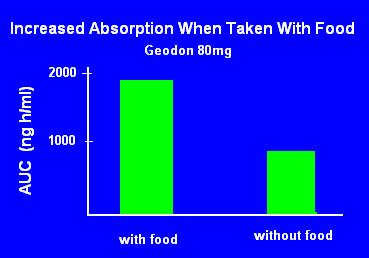
The FDA recommends test meals for medication studies, but specifically two: high fat (50% of calories from fat) and high calorie (1000 calories.)
It isn't clear from this slide what constituent of food is necessary to the absorption. Some foods need specific conditions for absorption (e.g Vitamin C needs an acidic environment and absorption is reduced by fat.)
Geodon is highly lipophilic, so it would make sense that fat is key to absorption. Nope:
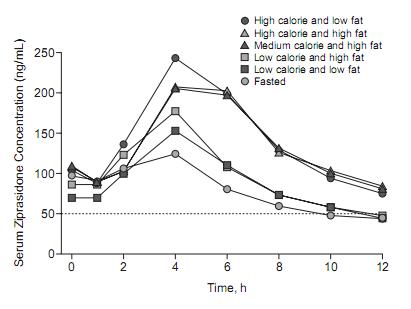 Low, medium and high calorie diets are 250, 500, or 1000, respectively.
Low, medium and high calorie diets are 250, 500, or 1000, respectively.
Fat appears to have little to do with it. Calories matter, thought the benefit appears to maximize at 500 calories-- doubling to 1000 calories is only marginally better.
What isn't known is whether the effect is due to pH, transit time, or some other factor.
The simple problem is that to get Geodon to work you need 120mg or so, and it has to be taken with food. Any claims about lack of efficacy before these two conditions are met are just plain silly. They should have made a slide with that, but they didn't.
Slide 12: Dosage Adjustments Are Not Necessary With Geodon
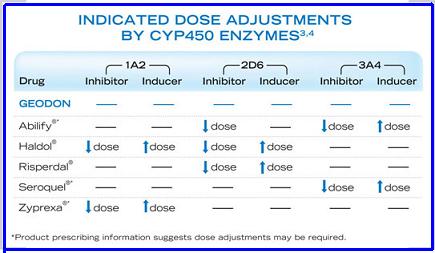 (I found this slide on the internet and saved myself an hour with MS Paint.)
(I found this slide on the internet and saved myself an hour with MS Paint.)
This slide is often presented with another showing specific drug inhibitors/inducers (ciprofloxacin, phenytoin, etc.)
While theoretically legitimate, it mostly doesn't have enough of an effect with antipsychotics to matter. So there's little point to learning it, though the idea that Geodon is metabolized differently and has little effect on/from liver metabolism is good to know. (But there is a cardiac effect with other QTc prolongers.)
A better way to use this information:
Smoking cigarettes can dramatically reduce some antipsychotic levels. It can cut Haldol in half, and reduce Zyprexa by 40%. But in ordinary practice, that doesn't matter-- you'd have instinctively given the patient more mg because he's not better. You may not realize that you did this because the drug level is lower (not that he is sicker), but ultimately it doesn't matter.
Where it does matter is a hospital, where patients aren't allowed to smoke: psychotic patients is stabilized on 10mg Zyprexa, he gets discharged and goes back to smoking a pack a day. See?
So what really matters is changes from the usual. It's important to know that a guy takes HCTZ and smokes 2pp/d, but it's just as important to know if that status changes.
In my opinion, every opportunity should be taken to lower dosages or stop medications. If a guy quits smoking for real, I try to cut his Zyprexa in half. "Really?" Yes, really. The liver tells me to. If a patient "shows frequent noncompliance" with a medication but otherwise seems stable, I don't encourage them to comply. I reduce it or, if it's an SSRI, stop it entirely.
Slide 13: FDA Warning
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. GEODON (ziprasidone) is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions].
First, this is a warning about dementia related psychosis, not about being elderly. An 80 year old man with bipolar disorder does not have this risk; a 55 year old with dementia related psychosis does. (More accurately, no studies have found a similar mortality in elderly patients without dementia related psychosis.)
Nor is it even clear what the risk is: as many of these patients died of cardiac events and infections, it can't be blamed on a direct brain effect.
The warning was based on deaths reported in the 17 trials (5000 patients, aver age 81) using the six atypicals. Importantly, these were short term studies: the increased risk of death happened in 6-12 weeks.
Two observational studies found conventionals had the same risk.
There are numerous questions to ask, I'll ask two you might not have thought of:
-----------
If you liked this (and enough people link to it or click to it), then I may do one for all slide decks/promotional materials, and put it into an email newsletter.
http://twitter.com/thelastpsych
My intention is not to do a Carlat "all medicines suck" style hit piece. I want to show how to do a powerful analysis of a PROMOTIONAL slide presentation, and also how to detect some of the typical tricks/misdirections. These are frequently used by academics as well.
These slides are for Geodon's new indication, Geodon as an adjunct to Lithium or Depakote for Maintenance of Bipolar Disorder. It is what doctors (will) see at promotional programs (e.g. dinners) and versions of these will become rep detail pieces (what they'll show doctors.)
I don't have the slides, but I did get a peek at them, so I've redrawn them from memory. Yes, memory.
Slide 1:

Indications ("antipsychotic") etc, are descriptors, not identifiers. 15 years ago Geodon was tested for schizophrenia, and found to be effective. Today, it is tested for maintenance bipolar; so we say "this antipsychotic is also effective for bipolar maintenance." Wrong. That's an accident of history. 15 years ago they could have first tested it for bipolar, and today done schizophrenia trials, and then we'd say, "this is a mood stabilizer that also treats psychosis." Both of those statements are empty. It is a chemical, it has utility, not identity.
Side 2:

What does the author want to be true?
This is the main slide, disguised as a throwaway. As the "introduction" it clarifies for you what is established already-- according to the presenter-- even though it may not actually be established. "As has already been established, Windows is a superior operating system."
Pfizer has decided to market Geodon for mild states, and emphasize it's better weight profile.
Their message is: "hey, even though you think Geodon is weak, nothing works as monotherapy. And we at least have an indication for maintenance."
It seems there's no way to manipulate this: two simple quotes. The second quote seems to follow from the first, i.e. because the rate of success of monotherapy is so low, therefore you need a combination. But, in fact, reference two doesn't attribute the prevalence of combination therapy to a lack of efficacy. The sentence preceding quote 2 is:
In recent years, the therapeutic armamentarium for bipolar disorder has expanded in terms of options. Hence, the clinical management of bipolar disorder now usually involves a combination...
There's combination out of necessity, and combination out of availability.
Slide 3:

Their message: "We took some manic/mixed bipolar patients, and randomized them to mood stabilizer alone vs. mood stabilizer + Geodon, for 6 months, to see which kept them stable (time to intervention) longer."
Let's work from the middle "Stabilization/Randomization" square. What were they stabilized with? Li or VPA, + Geodon. What were they randomized to? Either a continuation of that, or the Geodon was taken away. So the study doesn't measure maintenance efficacy, it measures the speed of relapse if you take one of the two medicines that got you stable away.
Go back one step to the first circle. What happened to some of the 580 people who couldn't get stabilized, after 16 weeks, on Li/VPA + Geodon? They didn't stay in the study. Same with those who had some adverse event. 50%, gone. So by the middle square, the study had effectively selected for those patients who could tolerate and respond to Geodon.
Continuing backwards, it appears that the study begins at the big green vertical line, but it doesn't. Reformatting the slide gives a more clear explanation of the events:

You now can see that there was one extra initial step. Real outpatients on any kind of medications were screened. If they were manic (MRS>14 or 18) then they were TAKEN OFF THEIR MEDS and given Li or VPA, at therapeutic level, for 2 weeks. Those patients who DID NOT IMPROVE on the monotherapy were then given Geodon as well, for 3-4 months, until stabilized.
This effectively screens out patients who respond to monotherapy. Consider: you already know that by the end of the study, the monotherapy patients will relapse faster, because monotherapy didn't work in the first place.
Aside: this may be a question of severity, but how is it possible to let more than 600 people stay manic for two weeks on one single medication, and not intervene?
Slide 4:

Their message: "Geodon + Li or VPA was superior to Li/VPA alone for preventing relapse."
More full message: "Geodon + Li or VPA significantly increased the probability of being relapse free."
Of the 240 patents who started the maintenance phase, only 138 finished. Kaplan-Meier analysis is a way of analyzing the probability of an event (in this case, being relapse free) even when there are dropouts for other reasons. It's probably more useful to simply say this:
[during the 6 months] intervention for a mood episode was required by 19.7% (25/127) of subjects receiving ziprasidone, compared with 32.4% (36/111) of subjects receiving placebo.
...median time to intervention for a mood episode for ziprasidone and placebo, respectively,was 43 days (7-165) and 26.5 days (2-140), among patients who required an intervention (n=61).
You might be tempted to say the following: "hey, Depakote or lithium alone wasn't that bad-- 70% probability of being relapse free. So?"
What would be awesome was to have a straight placebo arm (i.e. Li/VPA + Geodon vs. Li/VPA + placebo vs. no meds at all), to compare.
Though one shouldn't compare across studies, etc, etc, compare the above survival curves to those found in the famous year long Depakote maintenance study (Depakote vs. lithium vs. placebo monotherapy trial for prevention of mood episodes-- (done by the same guy, hey. would you look at that))

In that study, the same maintenance power of depakote and lithium wasn't better than placebo.
Slide 5: Dosing

This slide does not tell you the doses used to keep people stable for 6 months; it tells you the doses used to stabilize the patient, i.e. the acute mania dosing.
It is true that they were then kept on these doses, but you don't know if doubling the dose-- or halving the dose-- would have changed the outcome of the maintenance phase.
It is already established in the acute trials that it takes 120-160mg of Geodon ALONE to treat acute mania; perhaps the 50% that only needed 80mg here had some effect of the VPA/Li; or there was better attention to giving with food here; or the permitted use of Ambien and Ativan (<2mg) also helped. In any case, it seems reasonable to say that one should not expect any acute efficacy at all under 80mg.
Slide 6: Nonadherence

Looks like a throwaway; but it's the whole marketing message. Weight gain= Zyprexa/Seroquel; Sedation = Seroquel; Akathisia = Abilify as defined in the secret Big Pharma Marketing Playbook. They all use the same copy. (Geodon would be QTc.) The slide lets you know that this is why patients stop their meds, so be proactive...
First, the actual Guidelines reviewed studies that find that denial of illness and lack of efficacy as the main culprits. In fact, they write "there is not a robust association between medication side effects and adherence... a survey of 3000 patients found that side effects ranked 7th on a list of concerns..."
But those studies aren't the point: the Expert Consensus Guidelines aren't a review of studies, they are a 40 question survey given to 40 experts in bipolar. "What do you think is the standard of care? What's your favorite Dr. Who episode?" Also note that these experts don't routinely treat patients.
The Experts rated side effects as the main adherence issue.
The experts' ratings agreed with the findings in the literature about the importance of poor insight and lack of illness awareness, belief that medications are no longer needed, and lack of treatment efficacy as key factors that can contribute to adherence problems. It is interesting that the experts gave more prominence to side effects as a contributor to adherence problems than has been reported in surveys of patients and other studies in the literature.Yeah, that is interesting.
Even more interesting is which side effects these Experts rated as most important, in decreasing order:
- Weight gain (women)
- Sedation
- Sexual dysfunction (men)
- Cognitive side effects
- Weight gain (men)
- Sexual dysfunction (women)
- Akathisia
Final point:the studies found that patients were most troubled by weight gain and cognitive side effects:
It is interesting, although not surprising, that the experts considered excessive sedation a more important contributor to adherence problems for patients with bipolar disorder than schizophrenia, reflecting clinical experience that patients with bipolar disorder strongly dislike being sedated.It may very well be that Experts/academics see a population that doesn't like sedation, whereas an inner city psychiatrist might believe the only thing patients crave is sedation.
Slide 6: Weight Gain

It looks like Geodon had no effect on weight; alternatively, it looks like Li or VPA will cause 5% of patients to gain >7% of their body weight (e.g. 10-15lbs.)
But this tracks weight changes starting from the double blind phase. Remember, patients were loaded with VA or Li for two weeks, then Geodon was added for 10-16 weeks. Not to mention they were already on meds in their past. Could they all have gained 100 lbs in the first four months, only to level off in the maintenance phase? Of course. Did that happen? Who knows?
All that you can say is that after being on Geodon for 4 months, the proportion of people who go on to have even more weight gain in the next 6 months is 5%.
Slides 7 and 8: Sedation and Movement Disorders


Same deal: these are the side effects only in the last 6 months of a 10 month study. Could sedation have been massive but transient in the first four months? Could tolerance to the effects have developed? We know 50% of patients dropped out by the randomization phase-- half of those due to adverse events. Did the people who experienced sedation or movement disorders quit?
The study had effectively screened for patients who could tolerate these kind of side effects, so you'd expect them to be low in the last half of a study.
Slides 9 and 10: Discontinuation/Tolerability

Same deal, again.
Please take a moment and look at the slides as if it was your first time. Despite the clear labeling "At 6 Month Phase", you can't help but think "rates of adverse events were low."
If you're astute, you might even think, "weird, people on two medications actually had fewer side effects, lower discontinuation rates." And your mind would start speculating: well, the Geodon is actually a little activating, so it counteracted the sedation that was caused by the Depakote."
No. The Geodon doesn't counteract the sedation; nor are there really "low rates" of discontinuation. Remember, 50% of the people stopped the combo even before randomization, and 50% of those stopped specifically because of an adverse event.

So Slides 9 and 10 are showing you the rates of AEs in people who had already tolerated 4 months of both medications.
This is important and must be understood. Content-- whether it be a Pharma slide or a newspaper article or anything else-- is almost never factually inaccurate. But the story, the style, the presentation is intended to get you to lie to yourself. This slide very obviously says, "in the 6 Month Phase." There's no misinterpreting that-- and yet you did.
Don't blame yourself entirely, it is a trick. If the slides were presented with the intention of imparting information (instead of a story) then it would never have shown you only the Phase II data, it would have offered you something like this:
 which comes from the study itself.
which comes from the study itself.But don't get excited, the studies are almost never more honest than the Pharma slides. The above Subject Disposition tree is a very recent phenomenon in articles, forced on it by an exhausted readership, and many articles still don't use it. Not that it would make any difference: no one reads the articles anyway.
Slide 11: Geodon Must Be Taken With Food

The FDA recommends test meals for medication studies, but specifically two: high fat (50% of calories from fat) and high calorie (1000 calories.)
It isn't clear from this slide what constituent of food is necessary to the absorption. Some foods need specific conditions for absorption (e.g Vitamin C needs an acidic environment and absorption is reduced by fat.)
Geodon is highly lipophilic, so it would make sense that fat is key to absorption. Nope:
 Low, medium and high calorie diets are 250, 500, or 1000, respectively.
Low, medium and high calorie diets are 250, 500, or 1000, respectively.Fat appears to have little to do with it. Calories matter, thought the benefit appears to maximize at 500 calories-- doubling to 1000 calories is only marginally better.
What isn't known is whether the effect is due to pH, transit time, or some other factor.
The simple problem is that to get Geodon to work you need 120mg or so, and it has to be taken with food. Any claims about lack of efficacy before these two conditions are met are just plain silly. They should have made a slide with that, but they didn't.
Slide 12: Dosage Adjustments Are Not Necessary With Geodon
 (I found this slide on the internet and saved myself an hour with MS Paint.)
(I found this slide on the internet and saved myself an hour with MS Paint.)This slide is often presented with another showing specific drug inhibitors/inducers (ciprofloxacin, phenytoin, etc.)
While theoretically legitimate, it mostly doesn't have enough of an effect with antipsychotics to matter. So there's little point to learning it, though the idea that Geodon is metabolized differently and has little effect on/from liver metabolism is good to know. (But there is a cardiac effect with other QTc prolongers.)
A better way to use this information:
Smoking cigarettes can dramatically reduce some antipsychotic levels. It can cut Haldol in half, and reduce Zyprexa by 40%. But in ordinary practice, that doesn't matter-- you'd have instinctively given the patient more mg because he's not better. You may not realize that you did this because the drug level is lower (not that he is sicker), but ultimately it doesn't matter.
Where it does matter is a hospital, where patients aren't allowed to smoke: psychotic patients is stabilized on 10mg Zyprexa, he gets discharged and goes back to smoking a pack a day. See?
So what really matters is changes from the usual. It's important to know that a guy takes HCTZ and smokes 2pp/d, but it's just as important to know if that status changes.
In my opinion, every opportunity should be taken to lower dosages or stop medications. If a guy quits smoking for real, I try to cut his Zyprexa in half. "Really?" Yes, really. The liver tells me to. If a patient "shows frequent noncompliance" with a medication but otherwise seems stable, I don't encourage them to comply. I reduce it or, if it's an SSRI, stop it entirely.
Slide 13: FDA Warning
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. GEODON (ziprasidone) is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions].
First, this is a warning about dementia related psychosis, not about being elderly. An 80 year old man with bipolar disorder does not have this risk; a 55 year old with dementia related psychosis does. (More accurately, no studies have found a similar mortality in elderly patients without dementia related psychosis.)
Nor is it even clear what the risk is: as many of these patients died of cardiac events and infections, it can't be blamed on a direct brain effect.
The warning was based on deaths reported in the 17 trials (5000 patients, aver age 81) using the six atypicals. Importantly, these were short term studies: the increased risk of death happened in 6-12 weeks.
Two observational studies found conventionals had the same risk.
There are numerous questions to ask, I'll ask two you might not have thought of:
- The 17 trials were in 2002-5. Assuming Geodon was shown to have the same risk, how did it cause the risk if it was (probably) dosed low and without adequate meals? Is it therefore dose independent? Or is the mortality due to something else? Similarly, 5mg of Haldol is "more" antipsychotic than 5mg of Zyprexa, but less anticholinergic (etc.) If both of those carry the same risk (studies were not powered for this) then it suggests something else is the cause.
- I know these studies were mostly done in nursing homes. Were they mostly done by psychiatrists? Imagine an agitated patient gets a pill-- placebo or antipsychotic. Does the calming effect of the atypical delay a cardiology or infectious disease consult by a day or more... while the placebo patient appears sicker, so gets an EKG or CT faster? Remember, the average age is 81. How much time to you need to go from bad to dead? In other words, is it a function not of the medication, but of what I'm calling intervention bias: "we did this, let's see how it works before we do something else."
-----------
If you liked this (and enough people link to it or click to it), then I may do one for all slide decks/promotional materials, and put it into an email newsletter.
http://twitter.com/thelastpsych
26 Comments