May 2006 Monthly Archive
Ritalin Causes Cancer?
An eye-opening study from some Texans.
18 kids, newly diagnosed with ADHD, started the study, only 12 finished. They showed up on day 1, and blood was taken. The kids were then given Ritalin (methylphenidate) 20-54mg/d, as part of ordinary treatment, for three weeks. At the end of three weeks, another blood sample was taken. The bloods were evaluated for cytogenetic abnormalities.
In every single case, the frequency of chromosomal aberrations, sister chromatid exchanges (SCE), micronuclei, and nucleoplasmic bridges were all dramatically higher than at baseline. Not a little higher-- massively higher.
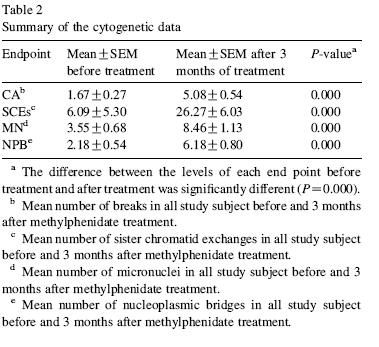
The authors had, in their introduction, summarized the absence of substantial evidence (or actually even studies) for carcinogenicity or mutagenicity, except one long term (2 year) high dose study in rodents-- it gave them hepatocellular carcinoma. But there has been nothing done in humans.
There are some problems with the study, beyond the obvious small sample size.
First, there's no control group. The assumption is that the only new factor over the three months of the study was the taking of Ritalin, so that is the likely culprit. Of course, it is certainly possible that something else occurred during those three months that could have caused this effect, such as a new illness, new meds, taking up smoking, etc. In all twelve people. At the same time. Sure, it's possible.
Second, the pretreatment group actually had less sister chromatid exhcanges than are expected on average. In a follow-up letter letter , the authors indicate that the known average frequencies of SCE are actually based on adults, not kids. Do kids have lower frequencies in general? Maybe.
The authors in that same letter also observe that despite the perception that there has not yet been on observed link between Ritalin and carcinogenicity, in fact
"the national toxicology program (NTP)—CERHR expert panel report on the reproductive and developmental toxicity of methylphenidate, indicate that only one study addressed the carcinogenic risk of methylphenidate treatment in humans... conducted by screening pharmacy and medical records, indicated that there was no increase in reports of cancer in a small number of patients taking methylphenidate (only 529 patients)."
I looked up the cytogenetic effects of amphetamines.
One study found methamphetamine exposure correlated to frequency of micronuclei and SCEs in humans (though, in hamsters, this effect was due exclusively to methamphetamine itself, and not its metabolites; and free radical scavengers also reduced this effect).
An old 2 year rodent study found decreases in number of neoplasms when given dl-amphetamine. Another study found a similar reduction, especially in pheochromocytomas, pituitary adenomas, and breast adenomas.
But again, these are rare studies, and this one here is the first done, prospectively, in humans.
What is astounding to me, apart from the obvious, is that no one knows this article. It has not been referenced in any subsequent articles. I can't find one psychiatrist, academic or otherwise, who has even heard this. They all look at me blankly: "Really?"
Yet, simultaneously, psychiatrists live with complete confidence that Ritalin is safe. They've never checked the known information before, of course, so what allows them to be so confident I have no idea; and they certainly don't run Medline once a month "just to keep up with all of science"-- but they're sure of what they know. Not even an empty patronizing nod to "but of course, our knowledge base is expanding..."
The point is not that Ritalin is unsafe. This study could be a load of crap, for all we know. But shouldn't psychiatry have at least heard of this study? What is the mechanism to disseminate this kind of information? How long does it take for something like this to hit the psychiatric press? In other words, given psychiatrists' arrogant confidence, how do they believe they would be informed of new developments? They don't really read psychiatry journals. They certainly aren't going to read cancer journals.
9/5/06 Update Further info suggests this may be a fluke.

 Score: 2 (2 votes cast)
Score: 2 (2 votes cast)
Liver and Medications
Here's how to think about the effect of the liver on drugs:
When you eat a drug, some of it gets bound to protein (albumin) and some circulates freely. Your body uses free, non-protein bound drug.
Most drugs are mostly protein bound-- notable exceptions are lithium, Ritalin, Lexapro (40%) and Effexor (40%). Low albumin-- as could occur in cirrhosis, severe malnutrition, etc-- increase the availability of the free form of the drugs. So low albumin + valium = more valium for you.
Next is volume of distribution (Vd)-- drugs with high Vd will diffuse into fluid spaces. So patients with a lot of edema will end up with lower useful drug (because it diffused into third spaces.) Be aware that diuresis may consequently increase the dose as it returns to circulation.
First pass metabolism is also important. First pass metabolism means that a substantial portion of the drug is metabolized quickly-- or, conversely, won't be metabolized if your liver is damaged. The appropriate doses of medications are based on functioning livers. For example, tricyclics are metabolized by 50% on first pass; which means, in the absence of a liver, you are actually giving twice as much drug as what you think you are prescribing. Zyprexa has 40% first pass. Dilantin, by contrast, has low first pass metabolism, so the dose is about the same.
There are two phases of liver metabolism.
Phase 1 occurs in smooth endoplasmic reticulum: reduction, oxidation, and hydrolysis. All the cytochrome P450 happens in Phase 1.
Phase 2 occurs in periportal region of portal triad: glucoronidation, acetylation, sulfation.
The trick of this is to understand that liver damage (cirrhosis, etc) affects Phase 1, not Phase 2.
This is why Ativan (lorazepam), Serax (oxazepam) and Restoril (temazepam)-- all metabolized primarily by Phase 2-- are favored in drinkers or cirrhotics. Also, renally metabolized or cleared drugs will not be as much affected (for example, Neurontin.)
I hope this was helpful. Please drink responsibly.

 Score: 3 (3 votes cast)
Score: 3 (3 votes cast)
Parenting and Personality: MAO-A
Continuing the series:
The authors investigate the interaction between child abuse and MAOA (gene) activity on future antisocial behavior.
FYI: MAOA is a gene on the X chromosome-- so males only have one copy. It makes MAO-A enzyme, which metabolizes serotonin, dopamine, and norepinephrine, so having low MAOA gene activity probably means less MAO-A, and thus more serotonin, norepinephrine, and dopamine. MAO-A is in CNS, liver, and GI tract; MAO-B (metabolizes mostly dopamine and phenethylamines (e.g. amphetamines)) is in CNS and platelets.
Importantly, having been maltreated in childhood predisposed you to becoming antisocial; having the MAO-A deficiency, by itself, did not. This is important: MAO activity has no effect in the absence of child abuse. Having low MAOA activity does not predisose you to violence. The abuse is the primary determinant.
What about the interaction between the environment (abuse) and biology (MAOA)? This interaction is very significant, but how you explain this interaction makes all the difference. Here's the figure:
The easy (and wrong) explanation, the one that jumps out at you, is this: if you were maltreated, having low MAO-A predisposes you to becoming antisocial.
But that's not what the figure shows. What it shows is that having high MAOA mitigated, i.e. lessened, the effect of being abused on future criminality.
What you see is that when MAO-A is high, you are protected against the effects of abuse. When it is low, abuse matters.
It may seem like the distinction between MAO being protective vs. being a risk factor is only semantics, but it isn't. How we define the problem actually generates different problems. "Having low MAOA increases your risk of being antisocial" is a very different social problem than "having high MAOA lessens the effect of child abuse." So if you're a lawyer, don't go concocting a "low MAOA made me do it" defense.
As an aside, it might be helpful if someone could explain how having low MAOA is a risk factor for agression and violence, but taking antidepressants (and MAO inhibitors especially) are supposed to make you less violent?

 Score: 4 (4 votes cast)
Score: 4 (4 votes cast)
Parenting and Personality Disorders
A fascinating article that no one will ever actually read: Parenting Behaviors Associated With Risk For Offspring Personality Disorder During Adulthood.
The authors made a (startling) discovery: there are types of parenting behaviors which predispose your kid to growing up personality disordered.
This was a longitudinal study of 592 families, first assessed when the kids were about 5, and then again when they were in their 30s. (More info at their website http://nyspi.org/childcom/)
The results are pretty much what you'd expect:
The more of these behaviors the parents exhibited, the more the risk of PD increased. What is interesting is which PD was increased given the number of parental behaviors:
First, overall number of bad parental behaviors:
(antisocial=criminal; avoidant=shy; narcissistic=self-absorbed)
You'll notice that antisocial PD is essentially zero at baseline, and is dramatically sensitive to bad parenting. Contrast this with avoidant PD, which, while also sensitive to the parenting, starts out higher at baseline. In other words, you may be born shy, but not antisocial.
Looking at specific types of bad parenting:
What you'll see in the top figure is that being an aversive parent is a great way of making someone borderline or passive-aggressive, not to mention paranoid. But it doesn't make them antisocial. Hmm.
Meanwhile, having low affection or low nurturing scores increased the risk for antisocial, as well as everything else (but especially avoidant, paranoid, depressive, borderline).
Some covariate caveats: even when parental psychaitric disorders and offspring behavioral problems at age 6 were controlled, bad parenting was still associatd with increased risk of their kids' PD.
Furthermore, the usual association of parental psychiatric disorder leading to child PD could be explained, in fact, 95% due to the bad parenting. Another way of saying this is that 95% of the effect that a parental psychiatric disorder has on causing their kids' personality disorder can be obviated by better parenting. In a similar vein, 35% of the effect of childhood behavioral problems leading to later PD can be similarly reduced by better parenting. In other words, even if you or your kids have a "biological" psychiatric disorder, better parenting skills can darmiatically affect the outcome.
It is not an insignificant fact that only one of the 5 authors was an MD (oddly, he is also a PhD but does not list this in the authorship line.). The nature vs. nurture debate in psychiatry is all but dead.
The longer we delude ourselves that biology controls behavior, and not the other way around, the longer we'll have to live with the same behaviors.

 Score: 12 (12 votes cast)
Score: 12 (12 votes cast)
Zyprexa's Weight Gain: Does What You Eat Matter More Than How Much?
The authors of this article have an interesting hypothesis, upon which I speculate wildly. But it is fascinating:
GLUT5, is found primarily in the small intestine (though also in muscle and kidneys.) What's interesting about it is that it transports fructose, which in turn directly stimulates additional GLUT5 mRNA expression. You eat fructose, this increases the expression of GLUT5 in the intestinal villi, which increases the transport of fructose. So the more fructose you eat, the more readily you can absorb it.
Now fructose doesn't stimulate insulin secretion. Since insulin regulates leptin, fructose actually reduces leptin. Fructose increases ghrelin. So you get hungry. Fructose goes to the liver and is metabilized to acyl glycerols, and consequently result in increased triglycerides.
So you have a situation in which Remeron and Zyprexa (and high dose Haldol) cause an increase in GLUT5 expression; if they are also eating fructose (read: high fructose corn syrup) this is causing an additional expression in GLUT5, and hunger, and increased triglycerides... if one wants to conduct a useful experiment, find out if the people who gain the most weight on Zyprexa are those who consume the most high fructose corn syrup (and not just those who eat the most.) In other words, can you gain weight on Zyprexa if you are eating Atkins?
(NB: there are many who want to believe that Zyprexa causes weight gain by increasing leptin; and so fructose and GLUT5 lowering leptin seems confusing. Zyprexa, as shown above, actually decreases leptin, acutely. (And clozaril has either no effect, or minimal lowering.) Letpin only increases with increased fat-- i.e. as a consequence of fat, not as the cause of fat. Those who have found increases in serum leptin do so only after chronic administration, and resultant weight gain (for example, in a study of 13 schizophrenics on Zyprexa who showed a small increase in leptin after 4 weeks-- and after a 2 kg weight gain; or 6 week animal study finding increased fat and leptin. The question, as noted by the authors, is whether the acute hypoleptinemia and hypoglycemia is what triggers hunger and an ultimate increase in fat, leptin, glucose and insulin. )

 Score: 2 (2 votes cast)
Score: 2 (2 votes cast)
How Do Antipsychotics Cause Weight Gain?
In order for this post-- and any discussion on antipsychotic induced weight gain-- to make sense, you have to understand one thing: each antipsychotic seems to cause weight gain by a different mechanism, not varying degrees of the same mechanism. Because let me tell you right off the bat: researchers here are far from agreed.
A review of some articles:
In rat pancreatic beta cells, neither clozapine nor haloperidol had any effect on basal insulin release. In the presence of high glucose, haloperidol had no effect on the normal insulin surge, but clozapine inhibited this effect by 40%. How it did this is not clear, as clozapine, in the presence of glucose, completely suppressed electrical activity by hyperpolarizing the membrane potential (i.e. increased K+ conductance.) Haloperidol depolarized (inhibited K+ conductance). Thus, by completely suppressing electrical activity, it should have completely suppressed insulin release-- but it only inhibited 40%. Similarly, haloperidol should have increased insulin release (via depolarization) but it didn't have any effect. We don't know what would have happened if the study had been continued for a year; but note here that the effect on insulin is dependent on the presence or absence of glucose, not the other way around.
Most studies focus on the changes in serum parameters (triglyceride, cholesterol, insulin, etc) and not the mechanism for these changes.
For example, in 112 schizophrenics on meds for 8 weeks, Zyprexa, clozapine, Risperdal, sulpiride all increased insulin and C reactive peptide, as well as insulin resistance; but only clozapine and Zyprexa increased triglycerides and cholesterol, and had a greater impact on insulin, insulin resistance, and C-peptide. What you can't tell is when this happened and what came first: did the insulin go up as a direct effect of the med, and consequently so did cholesterol, or did insulin resistance happen first, etc?
In the first study looking at the drugs' effects on GLUT1-5 mRNA, it was found that Remeron (mirtazapine) increases GLUT4 (muscle/fat) and 5(intestine) mRNA, and Haldol and Zyprexa increase GLUT5. No effect on GLUT1-3. (Contrast with Clozaril and Risperdal, below.)
The authors propose something interesting about Remeron: "Therefore, the increasing effects of mirtazapine on GLUT4 mRNA levels in our study might lead to a decrease in blood glucose levels and to an increase in cellular fat deposition, leading to intermittent or continuous lowering of blood glucose levels with a subsequent increased uptake of carbohydrates and other types of nutrients." In other words, better glucose uptake into cells means more fat inside cells, and less glucose outside cells (hypoglycemia)-- which is a stimulus to eat more.
This is important, so I'll repeat it: the hyperglycemia seen with Zyprexa and Remeron is here proposed to be due to the acute lowering of blood glucose (because of increased transport), and thus an increase in eating and fat deposition, and consequently insulin resistance and hyperglycemia; not a direct affect on glucose metabolism.
(Consistent with Zyprexa's effect on GLUT5 (and not on carbohydrate metabolism, per se), metformin did not prevent weight gain in 40 people on 10mg Zyprexa (all gained 5-6kg in 14 weeks.)In (male C57) mice, over a 6 month period, clozapine, chlorpromazine and quetiapine induced hyperglycemia via effects on glucose transport. Haldol and amisulpiride have little effect on GLUT, and were found not to induce hyperglycemia. Risperdal had a medium effect on hyperglycemia, but at the lower doses.
Using rat pheochromocytoma cells, clozapine and Risperdal both inhibited glucose transport (i.e. GLUT3).
Desmethylclozapine (a metabolite) was an even more potent inhibitor, while clozapine-N-oxide, the other metabolite, had no effect on glucose transport. Clozapine and fluphenazine also inhibited glucose transport in (rat) muscle cells. The drugs block glucose transport in a non-competitive (i.e. allosteric) manner (and tricyclics appear to work in the same way.) What is interesting about this is that different people metabolize clozapine differently, and perhaps those who create more desmethylclozapine get more hyperglycemia than those who make less (and/or more clozapine-N-oxide.
A follow-up study tried to correlate the toxicity of these drugs to cells to their inhibition of glucose transport.
They found that clozapine, desmethylclozapine, Seroquel and fluphenazine were toxic to cells; Risperdal was minimally toxic; and Zyprexa actually promoted cell growth.
Seroquel, Zyprexa and clozapine all inhibited glucose transport about the same amount, and in a dose dependent manner. (Remember: Haldol and sulpiride don't.)
However, if the cells were exposed to drug for a longer time, fluphenazine greatly inhibited glucose uptake, clozapine had no effect, and Zyprexa increased glucose uptake. In other words, the toxic typicals only need a sort exposure to kill a cell, while less toxic atypicals need prolonged exposure. Also, fluphenazine increased GLUT3, and the atypicals had little or no effect (as found above.)
Zyprexa was found not to affect either the basal or the insulin stimulated glucose transport via GLUT1 or 4. (Fun fact: bovine serum albumin (or impurities therein), used to replicate the fact that olanzapine is highly (93%) protein bound, actually increased basal glucose transport, making suspicious all studies previosuly done with BSA.) This contradicts the findins of the Dwyer articles, above, where antipsychotics had inhibitory effects on glucose transport. A possible explanation could be dosing: this study used doses comparable to 20mg, while others used 20x that amount.
Another study, in humans, found that neither Zyprexa nor Risperdal affected acute (3 week) insulin sensitivity. Again, what happens after you get heavy is up for debate.
So what we have here is confusion, but:
1. acute, high dose in vitro studies indicate that typicals>atypicals inhibit glucose transport, but Haldol does not.
1b. Typicals are toxic to cells, atypicals less so, and Zyprexa promotes cell proliferation.
2. Normal dose and human studies show no effect on insulin dependent glucose transport (i.e. GLUT4) but there are effects on small intestine absorption (GLUT5) with Zyprexa and Remeron.
3. Clozapine inhibits insulin release in the presence of glucose, but Haldol doesn't.
4. Acute effects may be different than chronic. i.e. even though antipsychotics may not directly affect insluin resistance or glucose transport, if they make you hungry or increase fat over time, this could result in later insulin resistance, hyperglycemia, etc.

 Score: 0 (2 votes cast)
Score: 0 (2 votes cast)
Atypicals and Diabetes: Glucose Transport
Glucose is absorbed through the small intestine into the blood.
All glucose is taken into cells via hexose transporters: this is facilitated diffusion (no ATP). Facilitated diffusion is passive diffusion through a channel made by a transmembrane protein; the proteins are able to open and close this channel. There are many ways channels can be opened/closed: ligand gated (i.e. neurotransmitter receptors), voltage gated (neurons), or, in the case of hydrophilic molecules such as glucose, mechanically gated: the channel is shaped like a closed "V". Glucose goes to the bottom of the V, causes a conformational change and the "V" opens, but closes at the top (makes an upside down "V".) Glucose can pass, and the V recloses. All diffusion is down a concentration gradient.
The hexose transporters are called, randomly, "Glucose transporters 1-5" (GLUT1-5).
GLUT4 is the main transporter in muscle, fat, and the heart. GLUT4 is insulin-sensitive (though it can also be activated by muscle contraction-- go figure.) In the absence of insulin, GLUT4s are stored in cytomplasmic vesicles floating around in the cytosol. If insulin binds to the insulin receptor (an ATP dependent tyrosine kinase receptor-- NOT the GLUT4), a signal cascade is activated that causes the cytpolasmic vesicle to go to and bind to the plasma membrane and lodge the GLUT4 there. The GLUT4 then allows glucose to diffuse through. When insulin disappears, the insulin receptor reconforms, the signal cascade stops, and the GLUT4 pinches off (by clathrin and other contracting proteins in the cell membrane) into a vesicle again (pinocytosis).
Thus, if there is no insulin: even if there is much glucose, there is no signal for the vesicle to go to the plasma membrane and lodge the GLUT4, so there will be no transport of glucose into the cell; so glucose stays high in the blood. Thus we have Type 1 diabetes.
Insulin also stimulates the creation of glycogen in the liver and muscle. [Insulin activates hexokinase (1st enzyme in glycolysis) as well as phosphofructokinase and glycogen synthase) and inhibits glucose-6-phosphatase ((opposite direction of hexokinase, same reaction) gluconeogenesis).]
Insulin promotes fatty acid synthesis.
Once glycogen synthesis has maxed out (i.e. about 30g, about 20% of the carbohydrate part of a studied meal, max in 4-6hrs,) then fatty acid synthesis IN THE LIVER takes over. Glucose is converted to free fatty acids (FFAs) and dumped back into the blood as lipoproteins-- which are then broken up into FFAs.
FFAs go into the adipose cells of the body. Glucose also goes into adipose cells-- via GLUT-- and are converted into glycerols. Glycerol+FFAs= triglycerides.
Thus, insulin's role is to store fat and/or oxidize glucose. Too little insulin will also trigger protein catabolism.
GLUT1 and GLUT3 account for 95% of the glucose transport to the brain. GLUT1 is for the blood brain barrier (the tight junctuions of the BBB are what require these channels), and GLUT3 is in the neurons. (pic here) GLUT1 is also found in muscle.
These are not insulin dependent (like GLUT4 is) so the brain can continue to get its energy. Not only does the distribution of GLUT 1 and 3 mirror capillary density and areas of relative glucose utilization, the GLUT1/3 densities can change depending on chronically increased (or decreased) need for glucose. Interestingly, nicotine, which increases brain glucose utilization, increases GLUT1/3 but not capillary density.
GLUT2 and 7 are in the liver. GLUT2 can also carry D-fructose.
GLUT5 is in the intestine, and some glial cells of the brain.
Type II diabetes is insulin resistance, not lack of insulin. There is not, at least initially, a problem with the pancreas's secretion of insulin in response to high glucose. The problem is at the level of the insulin receptor and/or GLUT, which become insensitive to the effects of insulin-- because there has been so much of it for so long. (For more info, see: News Physiol Sci. 2001 Apr;16:71-6.
Next up: how do antipsychotics affect glucose/insulin/transporters?
(For a review: What We Know About Facilitative Glucose Transporters )

 Score: 3 (5 votes cast)
Score: 3 (5 votes cast)
Modafinil vs. Clozaril
I'm researching modafinil for my last post, and bam-- I see this thing. How is no one talking about this article? Does no one read anymore?
A short intro to EEGs and antipsychotics.
Generally, antipsychotics increase power across all frequencies, but each drug (or receptor system) is associated with a specific frequency's power increase. Additionally, antipsychotics' effects are region specific: here, the prefrontal cortex gets the majority of the effect.
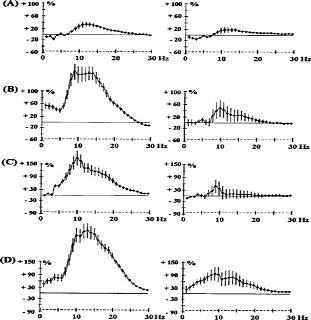
Effects of haloperidol 0.5 mg kg-1 s.c. (A) and 1 mg kg-1 s.c. (B); chlorpromazine 0.5 mg kg-1 i.p. (C) and quetiapine 2.5 mg kg-1 s.c. (D) on EEG spectral power in rats. Panels on the left show data from prefrontal cortex, panels on the right show data from sensorimotor cortex.
Everything is higher (i.e. above the line), but see how each drug or dose changes which frequency sees the most increase in power? So now you can make a comparison table:
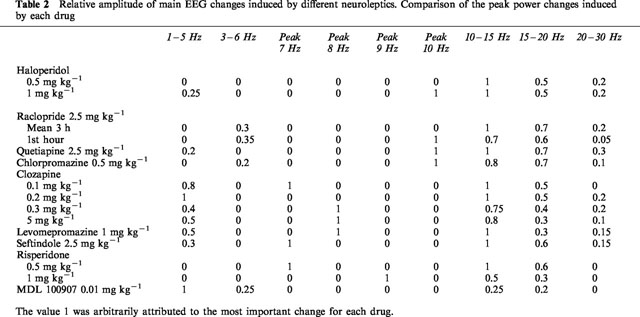
Summarizing:
D2 blockers (haldol, racloperide): increase in 10-15Hz power band (lesser in 15-20Hz)
Pure 5HT2A blockers (MDL100907): increases 2 Hz power band
High 5HT2/D2 ratios (Risperdal, sertindole): peak synchronization at 7-10 Hz.
In other words, mixing receptors gets you a mixed effect on EEG.
Additionally, drugs with high alpha-1 blockade (Clozapine, chlorpromazine, quetiapine) increase in 8-10 Hz power band.
You would guess that mixing an antipsychotic with apomorphine (a dopamine agonist) might decrease these effects, and mostly this would be right. There seems to be more effect on the sensorimotor cortex, but this isn't today's message.
What do you think would happen if you mixed Provigil and racloperide (D2 blocker) or clozapine? With racloperide, Provigil increased the power in the 10Hz band in both prefrontal and sensorimotor cortex-- a lot.
But apomorphine added to clozapine had no effect-- and Provigil added to clozapine decreased-- and at high doses almost totally extinguished-- the effect. This is the opposite of what happens in a pure D2 blocker!
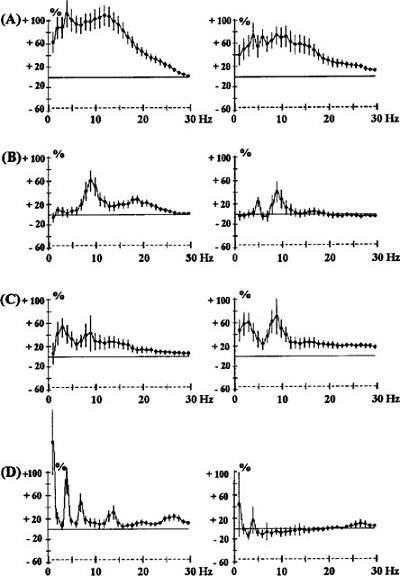
Effects of the co-administration of clozapine (CLZ, A) 0.2 mg kg-1 s.c. and modafinil (MOD) 62.5 mg kg-1 i.p. (B), 125 mg kg-1 i.p. (C) or 250 mg kg-1 i.p. (D) in rats. The ordinate represents the percentage change in EEG power. Vertical bars for each Hz show 95% confidence intervals. Panels on the left show data from prefrontal cortex, panels on the right show data from sensorimotor cortex.
The likely explanation is alpha-1 blockade. Clozapine is a potent alpha-1 blocker. The wakefulness promoting, and EEG, effects of Provigil, which has no affinity for adrenergic receptors, are strangely blocked by prazosin (an alpha-1 blocker.) Thus, the specific effects of clozapine on EEG synchronization must be through alpha-1, not dopamine (i.e. the opposite of racloperide), as evidenced by the fact that they can be negated by Provigil, but not by apomorphine (again, the opposite of racloperide.)
In other words, alpha-1 blockade is integral, not incidental, to the antipsychotic efficacy of clozaril. It's not just orthostasis. This bodes well for Seroquel as well. But the reason why alpha-1 blockade is important is not clear. More on this when I figure it out.

 Score: 1 (1 votes cast)
Score: 1 (1 votes cast)
Provigil vs. Cocaine
In an attempt to see if there is an interaction between cocaine and Provigil, 20mg or 40mg IV cocaine was given pre and post Provigil (400mg or 800mg) for 7 days. There was an interaction, but it turned out to be positive: Provigil reduced systemic cocaine exposure.
A safety study investigated (in 7 people) the interaction between cocaine (30mg IV) and Provigil (modafinil) 200mg or 400mg, or placebo, and found no synergistic effect on vital signs (T, BP, HR) or EKG. Not only did it not augment cocaine euphoria, it blunted it in one person.
In another study, 62 (mostly black) males addicted to cocaine were randomized to placebo, CBT, or Provigil 400mg. Abstinence, the primary outcome, was measured by benzoylecgonine in the urine. Patients on Provigil were abstinent longer, and produced fewer positive urines (i.e. fewer relapses.) Importantly, no one got addicted to Provigil.
Unlike cocaine and Ritalin (methylphenidate) Provigil did not produce "cocaine like discriminitive stimulus" (i.e. didn't feel like cocaine; Ritalin and cocaine do feel like cocaine.)
That's all we know about Provigil vs. cocaine so far, which is pitiful but not inconsequential. Given Provigil's near absence of terrible side effects, I say it's worth a try.
In the interest of completeness (and correctness) I have to correct the major paper, above (the 62 people with the urine tests) . The authors of that paper propose the following potential mechanism:
Its glutamate-enhancing action (Ferraro et al, 1998; 1999) might be clinically advantageous in cocaine dependence because the repeated administration of cocaine depletes extracellular glutamate levels
Except that the Ferraro paper doesn't actually say that. What it says is that it inhibits striatal and globus pallidus GABA, but doesn't directly affect glutamate. In order for it to have any effect on striatal glutamate, you needed 300mg/kg (i.e. 21,000mg. See you on the other side.) Given that GABA and glutamate are opposites (i.e. glutamate goes up because GABA goes down), it's probably a small point, but not an insignificant one: if it directly increases glutamate, it could antagonize Lamictal or even potentially cause seizures (and it does neither.)
The second Ferraro reference finds essentially the same thing: inhibition of medial preoptic area and posterior hypothalamus GABA, and consequently glutamate increases. And again, all of this occurs at preposterously high doses (100-300mg/kg.)
In interesting side finding of Ferraro's study is that the medial preoptic area and posterior hypothalamus are primarily controlled by tonic GABA inhibition; consequently modafinil's (or any drug's) effect of increasing glutamate in these areas can be blocked by giving a GABA-A antagonist.
So Provigil operates by (probably) by antagonizing GABA, not specifically by enhancing glutamate (neither synthesis of or transport of).
To further complicate this picture, it may be that the effects on GABA and glutamate are both indirect, and really the result of serotonin agonism. In an earlier study by the same guy, the decreases in GABA were partially prevented by a 5HT3 blocker (think Zofran, Remeron). Does Provigil work through serotonin? In a later study, the same guy finds that at 100mg/kg, Provigil does, after all, increase serotonin in the medial preoptic area and posterior hypothalamus. (At lower doses, 10-100mg/kg, it increases serotonin in the cortex, dorsal raphe and the amygdala.) (And in another study, (yes, by that same guy again,) 3mg/kg Provigil, which in itself has no effect on serotonin, synergistically augmented serotonin increase to fluoxetine and imipramine.)
We already know that Provigil can reduce the sedation that comes from varying drugs, like SSRIs, general anesthesia, haloperidol, and chlorpromazine. It would be interesting to see if Provigil was unable to improve sedation on Remeron, supporting the 5HT3 hypothesis.
Good luck out there.

 Score: 0 (2 votes cast)
Score: 0 (2 votes cast)
Are Antipsychotics Overprescribed In Kids?
According to USA Today, 2.5 million antipsychotic prescriptions a year are written for kids under 18. The rate for privately insured kids is 6.5 in 1000-- it has to be easily ten times that for Medicaid kids.
The FDA database has 45 deaths; 6 from diabetes, the rest from CV disease, liver failure, suicide, etc. There were 41 pediatric NMS cases.
According to the article, 13% of antipsychotic prescriptions are for bipolar disorder.
So are antipsychotics being overprescribed? The answer is yes, but not for the reasons cited in the article.
The article, indeed, all articles about pediatric psychiatry, make a special point about how these medicines are not FDA approved for kids. This is absolutely meaningless. FDA approval requires two double blind, placebo controlled studies. These studies are universally taken on by the drug companies. No drug company would ever assume the massive risk of such a study-- let a lone two-- in kids. How do you recruit the study subjects? What parent is going to allow it? Rich parents? No chance. So it will have to be Medicaid parents-- and thus will come the Tuskegee-like charges, dripping with the obvious social and racial implications of pharma testing on poor minorities. Pharma is already loathed; they're not going to take any risks for the sake of a medal from the FDA. So there will not be any new pediatric indications for psych meds. Not in this climate. Think this hurts Pharma? It's your kids that suffer.
But don't be confused by crypto-socialist hysterics who say that Pharma will do anything for a profit, including peddle drugs to kids. Drug companies do not market these antipsychotics for kids. They are paranoid to a fault about doing this; they know everyone is scrutinizing them, especially lawyers. If you are a child psychiatrist who sees no adults, reps cannot even call on you. And if they call on you for other things, they cannot mention the use in kids. In the past five years, it has never-- never-- happened that a rep detailed me about their use in kids.
The only two reasons these drugs are used in kids is because psychiatrists give them, and parents demand them.
First, the parents. They don't come looking for antipsychotics, specifically. But my experience is that they are unrealistic about what is going on with their kids; in near denial about the family dynamics impacting on the kid's behavior; and virtually devoid of insight into relatively obvious, though procedurally difficult, maneuvers that could improve the situation. If your kid doesn't sleep enough, and consistently-- if your five year old doesn't nap-- you cannot tell me your kid has ADHD. Period. Parents demand a diagnosis of bipolar disorder for their kids because it means the divorce had nothing to do with it. They demand another medication when the first one fails to get the kid to do math homework instead of playing Xbox all day. And their kids' marijuana and alcohol abuse can't possibly have anything to do with their own marijuana and alcohol abuse. Parents: don't flame me. Your situation is different, I know. I know.
Second, psychiatrists prescribe them because of the pressure to do something, in the face of consistent failure. They don't start with antispychotics-- they end up with them. They prescribe them out of desperation. This is why, in every story about a child getting sick from one of these medicines, they are, in fact, on several medicines. First they start with Ritalin. If Ritalin doesn't help, or there is a side effect, or they can't sleep-- then a second drug is added. Maybe this helps, but after a while something else happens-- and another drug is added to this. That's why psychiatry's current obssession with the detection of underdiagnosed "bipolar disorder" is so important. This diagnosis justifies, and encourages, polypharmacy.
It is psychiatry's ridiculously dangerous, and ultimately doomed, paradigm: if you are not doing well on a medication, you must be so sick that you require two medications. It seems to have occurred to no one in psychiatry that failure on a medication could mean that it was the wrong medication.
The reason this polypharmacy madness is even possible is psychiatry's obsession with diagnosis, labels-- with semiotics.
What makes a drug an antipsychotic? Well, it treats psychosis. Fine-- but does that exclude its efficacy for something else? If it is later found to be efficacious in, say, depression, then what do you call it? Is the drug an antipsychotic that's also good for depression, or an antidepressant that's also good for psychosis?
There's no value in the label "antipsychotic" or "antidepressant" except what we give it. It's a drug that treats psychosis and depression, not an antipsychotic that treats depression (or the other way around). If you can't see the difference, stop reading now and go back to watching American Idol.
For example, why are antipsychotics viewed as "off label" for kids? The word "antipsychotic" is meaningless. Antipsychotics are tested against a scale, like the Brief Psychatric Rating Scale. But these scales measure a lot of things, like depression, and not just psychosis.
And at what point did we start making a distinction between psychosis and "dementia related psychosis?" Or bipolar depression and regular depression? Why do we need separate FDA approvals? Does someone know something about the physiology of these disorders that I don't? Do we need to start approvals for "diabetes related depression?"
Saying an antipsychotic is worse than an antidepressant for depression is a valueless statement, especially in the absence of data on this question. You are actually better off asking, "which is better for depression, blocking the serotonin transporter or blocking 5HT2a receptors?" See? Put this way the distinction seems less obvious. And even that question is valueless, as there is nothing (that we know of at this time) that allows us to say what effect either pharmacologic maneuver actually has. 5HT2A blockade does what again? Really? Do you have any evidence for that at all? And no more post hoc ergo propter hoc nonsense. David Hume laughs at you.
A Simpson's reference is helpful here:
Homer: Not a bear in sight. The Bear Patrol must be working like a
charm.
Lisa: That's spacious reasoning, Dad.
Homer: Thank you, dear.
Lisa: By your logic I could claim that this rock keeps tigers away.
Homer: Oh, how does it work?
Lisa: It doesn't work.
Homer: Uh-huh.
Lisa: It's just a stupid rock.
Homer: Uh-huh.
Lisa: But I don't see any tigers around, do you?
[Homer thinks of this, then pulls out some money]
Homer: Lisa, I want to buy your rock.
I know. The FDA, the Scientologists, socialists, the parents at the end of their ropes- the easy thing to do is blame Pharma. I'm in the strange position of having to be a Pharma apologist, to be the only doctor willing to defend Pharma. There are plenty things I don't like about the way Pharma conducts business, but I can't voice these complaints because I have to use the time countering these inane attacks. I know what will happen if the Pharma critics get their way.
You think Pharma should have no sales contact with physicians? Fine. Now deal with the consequences.

 Score: 3 (5 votes cast)
Score: 3 (5 votes cast)
For more articles check out the Archives Web page ››