March 14, 2010
Swallow This: How Seroquel XR Works, Part 3

if you could only see the truth
Part 1
Part 2
Now let's stop asking how, and ask what: what does this drug actually do?
FDA:
Major Depressive Disorder sNDA Submission
The FDA approval of SEROQUEL XR for MDD was based on a supplemental new drug application (sNDA) comprising findings from two Phase III, placebo-controlled studies that assessed the efficacy and safety of once-daily treatment with SEROQUEL XR as adjunctive treatment in patients with MDD.
Note the word adjunctive. Two trials showed its efficacy as an adjunct. Like Abilify, Seroquel is only indicated for use as a supplement to a failed/failing antidepressant. Maybe it's a necessary combination of a serotonergic effect plus (a non-existent) noradrenergic effect, or some other synergistic mechanism; but it's an add-on, so get adding.
An adjunct-- to what?
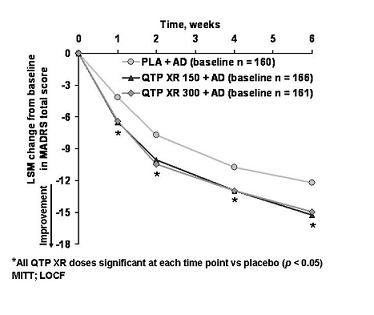
There's your data, it's one of the two trials the FDA used to grant the indication. The other trial's data was nearly identical. (1)
Seroquel was tested as an add-on to SSRIs, and also Effexor and Cymbalta. (The top line is placebo + SSRI/SNRI.) If we believe that at the doses used Effexor and Cymbalta are blocking the NET, and Seroquel at 150mg is doing the same, then why would we expect the addition of Seroquel to be of any use? They're both fighting for the same site, only one can bind, so? You wouldn't mix Paxil and Zoloft, would you?
So either the NET isn't relevant here; or, if it is, then those patients on Effexor/Cymbalta + Seroquel will not show greater improvement (e.g. as compared to Celexa + Seroquel). And the pooled data you see above will appear less impressive because the Effexor and Cymbalta patients dragged the overall average down.
Or, more simply: if they did the study only with SSRIs, maybe the augmentation results would have been even better!
So either the FDA is wrong you shouldn't mix it with Effexor/Cymbalta, or you should because the FDA is wrong about the mechanism. Good luck, have a drink. Your next patient is the dumpster guy from Mulholland Drive.
You're frightening me with your talk of dumpsters and intimations of rum.
Drink up.
This is from the promotional slide deck that Astra Zeneca uses to detail doctors, showing the results of the two trials.
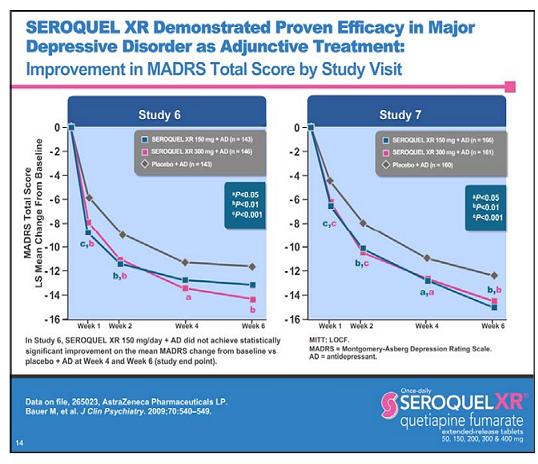
Take a good look at this slide, and compare it to the one above, from which it came. Do you see anything weird? Take minute.
Stop looking at the data curves. Stop looking at the p values. What do you expect to see there? You already know it's going to beat placebo or else they wouldn't have made a slide. Do you really think you're going to discover something there?
This slide is an illusion like the impossible fork, you want to see this as a representation of something else instead of seeing it for what it is.
I'll give you a hint:
What happened to studies 1-5?
Undoubtedly, your first thought will be that AZ hid the data. I wish that was true.
This is a promotional slide. The purpose of it is to push Seroquel. That means that nothing on it is not either compelled by the FDA or on purpose to get you to believe. They are accurately labeled studies 6 and 7 so that there so doctors ask about the other 5 studies. Why do they need doctors to ask? Because the FDA forbids them from mentioning them. So strict is this ban that the AZ reps are not even told by the company there are 5 other studies, so that they don't accidentally mention them. I am not exaggerating. The reps don't know.
You know what makes me happiest? When the government considers certain information so disruptive that it not only doesn't it tell the citizens, it doesn't even tell other members of the government. So that the only people who know are the heads of the government, and the heads of a for-profit corporation.
There's a word for that kind of thing. But anyway.
So what were studies 1-5?
Studies 1-5 were all monotherapy studies-- specifically, 4 acute trials and one 6 month maintenance study. And all but one acute study worked.(2)
More importantly, two of the trials that worked used 50mg.
In fact, AZ also submitted 5 other studies-- all positive-- to the FDA showing efficacy in GAD at doses starting at 50mg.
So this drug is efficacious as monotherapy in depression and anxiety?
Apparently so, and apparently at doses less than 150. Of course, since the adjunct trials were only done using 150 and 300, those are the doses that get approved. Safety, to the FDA, means they'd rather you get 3x to 6x the necessary dose-- along with a probably irrelevant drug-- than reveal that it worked as monotherapy.
Wait a second-- if there's very little NET inhibition at 150mg, doesn't that probably mean there isn't any at all at 50mg?
Oops.
Why would Astra Zeneca want to convince us that the mechanism of antidepressant effect was NET inhibition, when there is so much evidence (that presumably they are aware of) that it isn't?
Astra Zeneca doesn't want to convince us of that: the FDA does.
----
Coming soon: Part 4
1. Note that 300mg was not better than 150mg. And why would it be? The higher the dose, the more dopamine blockade your getting, which isn't relevant to depression otherwise we'd be augmenting with Haldol, which we aren't. (NB: which is why Abilify augmentation stays below 15mg.)
2. Seroquel 150 vs. Celexa 20 vs. placebo: no difference.
---
http://twitter.com/thelastpsych
35 Comments